Tandem on nüüd ametlikult CE-märgistatud meditsiiniseadmena vastavalt MDR-ile
Loe lähemalt →

Company
About us
September 3, 2025
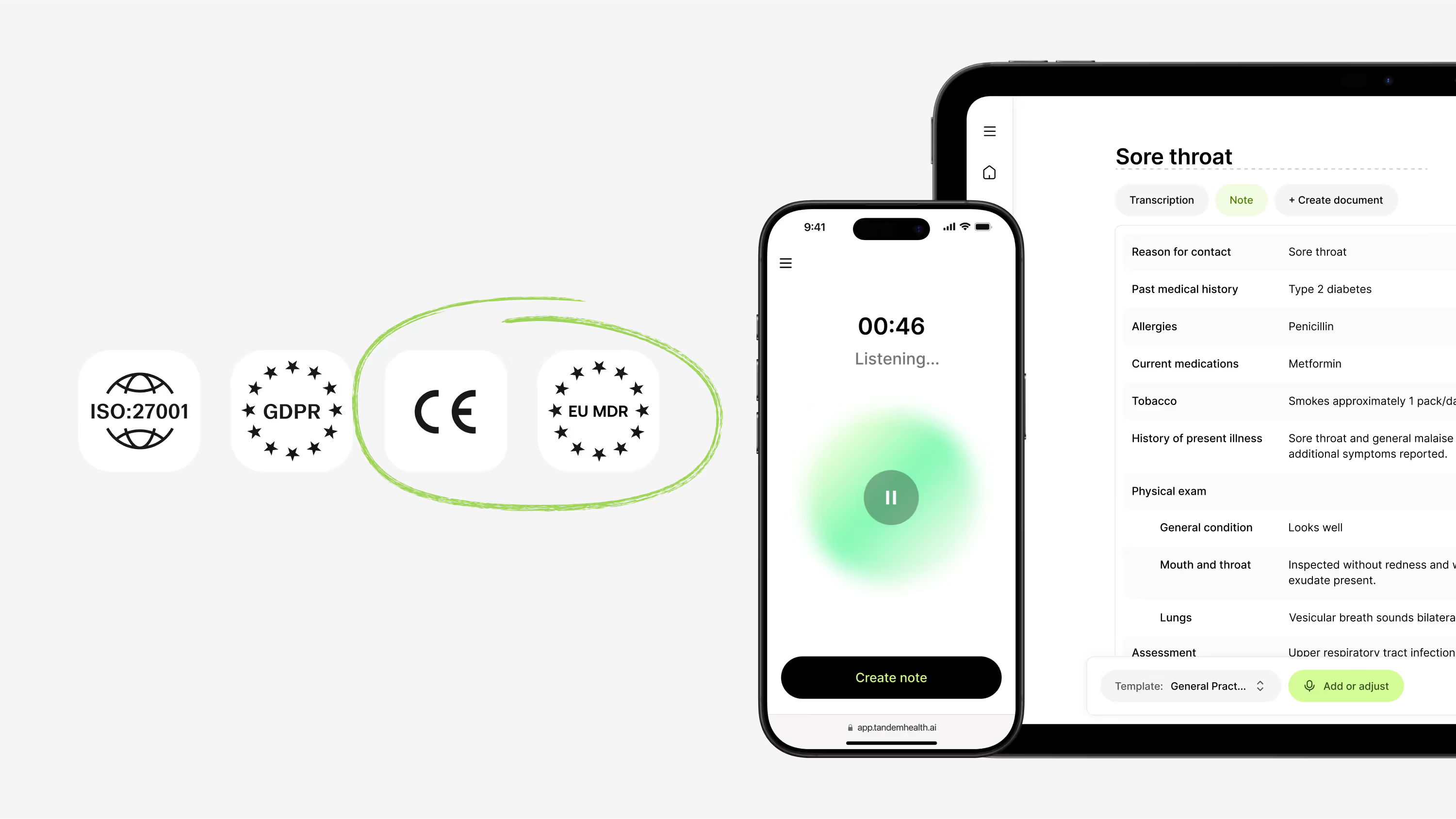
In our last post, we explained why AI scribes need to fall under the EU’s Medical Device Regulation (MDR). That shift matters because once software shapes patient records, it directly affects care.
Now we’re looking at what MDR compliance means in practice. Tandem’s AI scribe is officially CE marked as a medical device under MDR. For clinicians, that certification is more than a legal box to tick. It is a safeguard that every note and every feature is designed, tested, and monitored to the same standards as the other trusted tools in the clinic.
When AI shapes medical records, it stops being productivity software. In Europe, that makes it a medical device, and subject to Medical Device Regulation (MDR 2017/745).
The EU MDR is widely recognised as the most rigorous medical device framework in the world. It covers any software that generates or shapes medical records used in care, classifying it under Rule 11 of Annex VIII.
For AI scribes, this distinction is critical. If the software produces notes that become part of the patient file, it influences diagnosis and treatment. MDR ensures that such tools are subject to strict evaluation, risk management, and post-market surveillance.
Tandem’s AI medical scribe is officially CE marked as a Class I medical device under EU MDR. This recognition confirms that Tandem is regulated healthcare technology, not unregulated productivity software.
Certification is not abstract. It affects how clinicians work every day.
AI scribes are expanding quickly, but not all are regulated. MDR compliance is the difference between consumer productivity tools and medical-grade technology.
By aligning with MDR, Tandem shows that AI in healthcare can scale responsibly, keeping clinicians in control and patient safety at the centre. AI medical scribes can be developed very differently with varying degrees of quality checks and safety mechanisms in place. MDR provides an external validation that the product has been developed to meet safety and performance requirements.
MDR sets the foundation for safe medical devices. For AI scribes like Tandem, this means meeting clear standards of safety, oversight, and accountability.
For clinicians, the takeaway is simple: Tandem is not just helpful. It’s a regulated, reliable medical device you can trust every day.
If you want to understand why AI scribes must fall under MDR in the first place, see our earlier post: Why AI scribes need to fall under EU MDR.
Short answers about CE marking and MDR:
CE marking shows that a medical device meets EU MDR requirements for safety and performance. It also triggers post-market monitoring, giving clinicians ongoing protection.
Class I devices are considered low risk but must still meet MDR standards. AI scribes like Tandem fall under this category when they generate clinical records without replacing clinical judgment.
Because AI scribes directly influence patient records and care. MDR oversight ensures they are safe, transparent, and accountable – with requirements for usability, monitoring, and incident reporting.
Read more
No card details needed. Get started in 5 minutes.